Development of Modified Vaccinia Virus Ankara-Based Vaccines: Advantages and Applications
Abstract
:1. Introduction
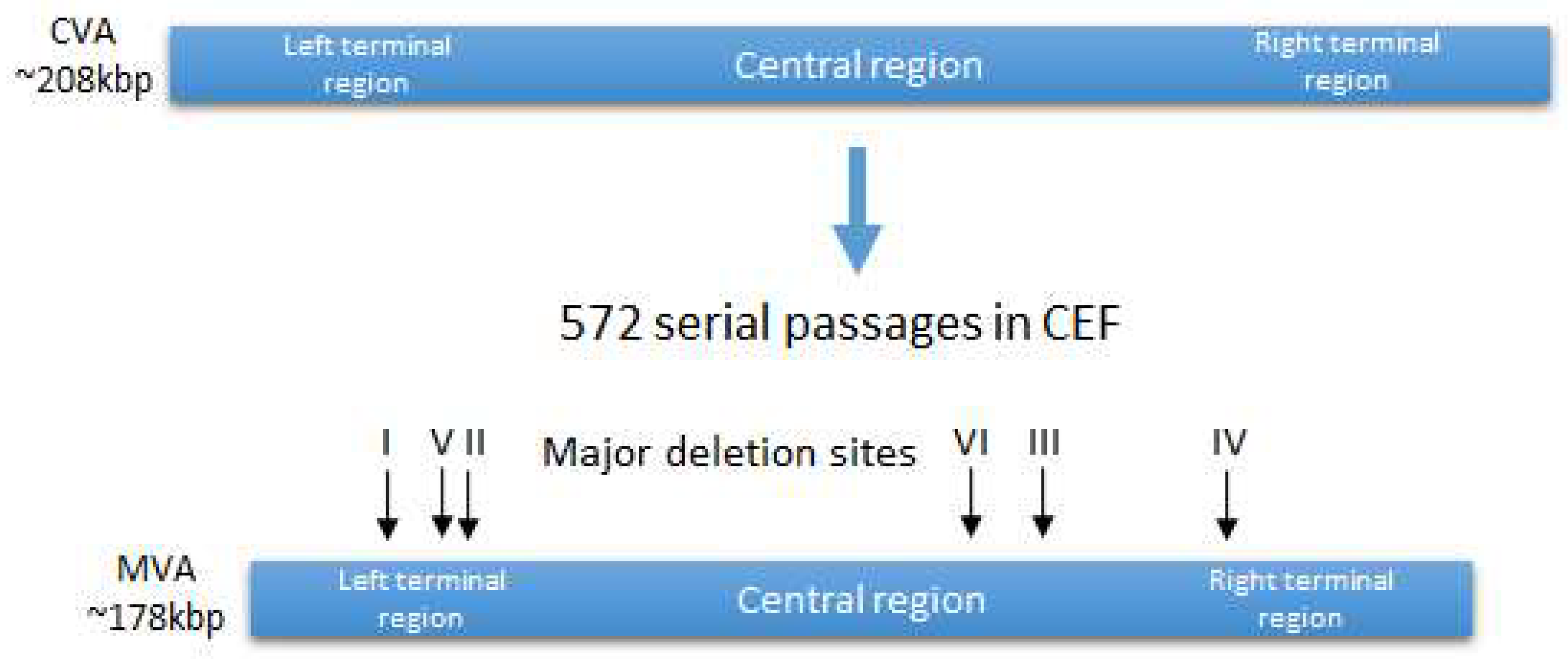
2. History of the Origin of the Vaccinia Strain MVA and Its Properties
3. Safety of Smallpox Vaccines Based on the MVA Strain
4. Recombinant Vaccines Based on MVA
5. Immunogenicity and Efficiency of Vaccines Based on MVA Vector
6. Preexisting Vector Immunity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I.D. Smallpox and Its Erradication; World Health Organization: Geneva, Switzerland, 1988; pp. 1–1460. [Google Scholar]
- Sánchez-Sampedro, L.; Perdiguero, B.; Mejías-Pérez, E.; García-Arriaza, J.; Di Pilato, M.; Esteban, M. The evolution of pox-virus vaccines. Viruses 2015, 7, 1726–1803. [Google Scholar] [CrossRef]
- Kaynarcalidan, O.; Mascaraque, S.M.; Drexler, I. Vaccinia Virus: From Crude Smallpox Vaccines to Elaborate Viral Vector Vaccine Design. Biomedicines 2021, 9, 1780. [Google Scholar] [CrossRef]
- Orenstein, W.; Offit, P.; Edwards, K.M.; Plotkin, S. Plotkin’s Vaccines, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Belongia, E.A.; Naleway, A.L. Smallpox Vaccine: The Good, the Bad, and the Ugly. Clin. Med. Res. 2003, 1, 87–92. [Google Scholar] [CrossRef]
- Kennedy, R.B.; Ovsyannikova, I.; Poland, G.A. Smallpox vaccines for biodefense. Vaccine 2009, 27, D73–D79. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Langland, J.O.; Kibler, K.V.; Denzler, K.L.; White, S.D.; Holechek, S.A.; Wong, S.; Huynh, T.; Baskin, C.R. Vaccinia virus vaccines: Past, present and future. Antivir. Res. 2009, 84, 1–13. [Google Scholar] [CrossRef]
- Kenner, J.; Cameron, F.; Empig, C.; Jobes, D.V.; Gurwith, M. LC16m8: An attenuated smallpox vaccine. Vaccine 2006, 24, 7009–7022. [Google Scholar] [CrossRef]
- Tagaya, I.; Kitamura, T.; Sano, Y. A New Mutant of Dermovaccinia Virus. Nature 1961, 192, 381–382. [Google Scholar] [CrossRef]
- Sánchez-Sampedro, L.; Gómez, C.E.; Mejías-Pérez, E.; Pérez-Jiménez, E.; Oliveros, J.C.; Esteban, M. Attenuated and replica-tion-competent vaccinia virus strains M65 and M101 with distinct biology and immunogenicity as potential vaccine candidates against pathogens. J. Virol. 2013, 87, 6955–6974. [Google Scholar] [CrossRef]
- Tartaglia, J.; Perkus, M.E.; Taylor, J.; Norton, E.K.; Audonnet, J.-C.; Cox, W.I.; Davis, S.W.; Van Der Hoeven, J.; Meignier, B.; Riviere, M.; et al. NYVAC: A highly attenuated strain of vaccinia virus. Virology 1992, 188, 217–232. [Google Scholar] [CrossRef]
- Meyer, H.; Sutter, G.; Mayr, A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. Gen. Virol. 1991, 72, 1031–1038. [Google Scholar] [CrossRef]
- Nájera, J.L.; Gomez, C.E.; Domingo-Gil, E.; Gherardi, M.M.; Esteban, M. Cellular and Biochemical Differences between Two Attenuated Poxvirus Vaccine Candidates (MVA and NYVAC) and Role of the C7L Gene. J. Virol. 2006, 80, 6033–6047. [Google Scholar] [CrossRef]
- Mayr, A.; Munz, E. Changes in the vaccinia virus through continuing passages in chick embryo fibroblast cultures. Zent. Bakteriol Orig 1964, 195, 24–35. [Google Scholar]
- Mayr, A.; Hochstein-Mintzel, V.; Stickl, H. Passage history, properties and applicability of the attenuated vaccinia virus strain MVA. Infection 1975, 3, 6–14. [Google Scholar] [CrossRef]
- Mayr, A.; Stickl, H.; Müller, H.K.; Danner, K.; Singer, H. The smallpox vaccination strain MVA: Marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism. Hyg. Betr. Prav. Med. 1978, 167, 375–390. [Google Scholar]
- Antoine, G.; Scheiflinger, F.; Dorner, F.; Falkner, F. The complete genomic sequence of the modified vaccinia ankara strain: Comparison with other orthopoxviruses. Virology 1998, 244, 365–396. [Google Scholar] [CrossRef]
- Volz, A.; Sutter, G. Modified Vaccinia Virus Ankara: History, Value in Basic Research, and Current Perspectives for Vaccine Development. Adv. Virus Res. 2017, 97, 187–243. [Google Scholar]
- Blanchard, T.J.; Andrea, P.; Alcami, A.; Smith, G.L. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: Implications for use as a human vaccine. J. Gen. Virol. 1998, 79, 1159–1167. [Google Scholar] [CrossRef]
- Liu, R.; Mendez-Rios, J.D.; Peng, C.; Xiao, W.; Weisberg, A.S.; Wyatt, L.S.; Moss, B. SPI-1 is a missing host-range factor re-quired for replication of the attenuated modified vaccinia Ankara (MVA) vaccine vector in human cells. PLoS Pathog 2019, 15, e1007710. [Google Scholar] [CrossRef]
- Peng, C.; Moss, B. Repair of a previously uncharacterized second host-range gene contributes to full replication of modified vaccinia virus Ankara (MVA) in human cells. Proc. Natl. Acad. Sci. USA 2020, 117, 3759–3767. [Google Scholar] [CrossRef]
- Sutter, G.; Moss, B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 1992, 89, 10847–10851. [Google Scholar] [CrossRef]
- Carroll, M.W.; Moss, B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: Propaga-tion and generation of recombinant viruses in a nonhuman mammalian cell line. Virology 1997, 238, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Drexler, I.; Wahren, B.; Sutter, G.; Heller, K.; Erfle, V. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J. Gen. Virol. 1998, 79, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Okeke, M.I.; Nilssen, Ø.; Traavik, T. Modified vaccinia virus Ankara multiplies in rat IEC-6 cells and limited production of mature virions occurs in other mammalian cell lines. J. Gen. Virol. 2006, 87, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H. Summary Report on First, Second and Third Generation Smallpox Vaccines; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Frey, S.E.; Newman, F.K.; Kennedy, J.S.; Sobek, V.; Ennis, F.A.; Hill, H.; Yan, L.K.; Chaplin, P.; Vollmar, J.; Chaitman, B.R.; et al. Clinical and immunologic responses to multiple doses of IMVAMUNE® (Modified Vaccinia Ankara) followed by Dryvax® challenge. Vaccine 2007, 25, 8562–8573. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.S.; Greenberg, R.N. IMVAMUNE®: Modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev. Vaccines 2009, 8, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.R.; Dolin, R. Vaccinia viruses: Vaccines against smallpox and vectors against infectious diseases and tumors. Expert Rev. Vaccines 2011, 10, 1221–1240. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Tchitchek, N.; Joly, C.; Stimmer, L.; Hocini, H.; Dereuddre-Bosquet, N.; Beignon, A.-S.; Chapon, C.; Levy, Y.; Le Grand, R.; et al. Molecular and Cellular Dynamics in the Skin, the Lymph Nodes, and the Blood of the Immune Response to Intradermal Injection of Modified Vaccinia Ankara Vaccine. Front. Immunol. 2018, 9, 870. [Google Scholar] [CrossRef]
- Slifka, M.K. The Future of Smallpox Vaccination: Is MVA the key? Med. Immunol. 2005, 4, 2. [Google Scholar] [CrossRef]
- FDA Approves First Live, Non-Replicating Vaccine to Prevent Smallpox and Monkeypox. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-live-non-replicating-vaccine-prevent-smallpox-and-monkeypox (accessed on 22 June 2022).
- Greenberg, R.N.; Hurley, M.Y.; Dinh, D.V.; Mraz, S.; Vera, J.G.; Von Bredow, D.; Von Krempelhuber, A.; Röesch, S.; Virgin, G.; Arndtz-Wiedemann, N.; et al. Correction: A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18–40 year old subjects with diagnosed atopic dermatitis. PLoS ONE 2015, 10, e0142802. [Google Scholar] [CrossRef]
- Overton, E.T.; Stapleton, J.; Frank, I.; Hassler, S.; Goepfert, P.A.; Barker, D.; Wagner, E.; von Krempelhuber, A.; Virgin, G.; Meyer, T.P.; et al. Safety and Immunogenicity of Modified Vaccinia Ankara-Bavarian Nordic Smallpox Vaccine in Vaccinia-Naive and Expe-rienced Human Immunodeficiency Virus-Infected Individuals: An Open-Label, Controlled Clinical Phase II Trial. Open Forum Infect. Dis. 2015, 2, ofv040. [Google Scholar] [CrossRef]
- Von Sonnenburg, F.; Perona, P.; Darsow, U.; Ring, J.; von Krempelhuber, A.; Vollmar, J.; Roesch, S.; Baedeker, N.; Kol-laritsch, H.; Chaplin, P. Safety and immunogenicity of modified vaccinia Ankara as a smallpox vaccine in people with atop-ic dermatitis. Vaccine 2014, 32, 5696–5702. [Google Scholar] [CrossRef] [PubMed]
- Overton, E.T.; Lawrence, S.J.; Wagner, E.; Nopora, K.; Rösch, S.; Young, P.; Schmidt, D.; Kreusel, C.; De Carli, S.; Meyer, T.P.; et al. Immunogenicity and safety of three consecutive production lots of the non repli-cating smallpox vaccine MVA: A randomised, double blind, placebo controlled phase III trial. PLoS ONE 2018, 13, e0195897. [Google Scholar]
- Overton, E.T.; Lawrence, S.J.; Stapleton, J.T.; Weidenthaler, H.; Schmidt, D.; Koenen, B.; Silbernagl, G.; Nopora, K.; Chaplin, P. A randomized phase II trial to compare safety and immunogenicity of the MVA-BN smallpox vaccine at various doses in adults with a history of AIDS. Vaccine 2020, 38, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sánchez, P.J.; et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preex-posure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States. Morb. Mortal. Wkly. Rep. 2022, 71, 734–742. [Google Scholar] [CrossRef]
- Earl, P.L.; Americo, J.L.; Wyatt, L.S.; Eller, L.A.; Whitbeck, J.C.; Cohen, G.H.; Eisenberg, R.J.; Hartmann, C.J.; Jackson, D.L.; Kulesh, D.A.; et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 2004, 428, 182–185. [Google Scholar] [CrossRef]
- Kretzschmar, M.; Wallinga, J.; Teunis, P.; Xing, S.; Mikolajczyk, R. Frequency of adverse events after vaccination with dif-ferent vaccinia strains. PLoS Med. 2006, 3, e272. [Google Scholar]
- Wiser, I.; Balicer, R.D.; Cohen, D. An update on smallpox vaccine candidates and their role in bioterrorism related vaccination strategies. Vaccine 2007, 25, 976–984. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Update: Adverse events following civilian smallpox vaccination--United States. MMWR Morb. Mortal. Wkly. Rep. 2004, 53, 106–107. [Google Scholar]
- Gurvich, E.B.; Vilesova, I.S. Vaccinia virus in postvaccinal encephalitis. Acta Virol. 1983, 27, 154–159. [Google Scholar]
- Rockoff, A.; Spigland, I.; Lorenstein, B.; Rose, A.L. Postvaccinal encephalomyelitis without cutaneous vaccination reaction. Ann. Neurol. 1979, 5, 99–101. [Google Scholar] [CrossRef]
- Zhang, C.X.; Sauder, C.; Malik, T.; Rubin, S.A. A mouse-based assay for the pre-clinical neurovirulence assessment of vac-cinia virus-based smallpox vaccines. Biologicals 2010, 38, 278–283. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, L.H.; Larkin, B.D.; Martin, J.E.; Graham, B.S. Modified Vaccinia Ankara: Potential as an Alternative Smallpox Vaccine. Clin. Infect. Dis. 2004, 38, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.T.; Jentzsch, U.; Metzger, E.; Simon, J. Studies on poxvirus infections in irradiated animals. Arch Virol. 1980, 64, 247–256. [Google Scholar] [CrossRef]
- Arness, M.K.; Eckart, R.E.; Love, S.S.; Atwood, J.E.; Wells, T.S.; Engler, R.J.M.; Collins, L.C.; Ludwig, S.L.; Riddle, J.R.; Grabenstein, J.D.; et al. Myopericarditis following Smallpox Vaccination. Am. J. Epidemiol. 2004, 160, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Elizaga, M.L.; Vasan, S.; Marovich, M.A.; Sato, A.H.; Lawrence, D.N.; Chaitman, B.R.; Frey, S.E.; Keefer, M.C. MVA cardiac safety working group prospective surveillance for cardiac adverse events in healthy adults receiving modified vaccinia ankara Vaccines: A systematic review. PLoS ONE 2013, 8, e54407. [Google Scholar] [CrossRef] [PubMed]
- Parrino, J.; Graham, B.S. Smallpox vaccines: Past, present, and future. Basic Clin. Immunol. 2006, 118, 1320–1326. [Google Scholar] [CrossRef]
- Zitzmann-Roth, E.-M.; Von Sonnenburg, F.; De La Motte, S.; Arndtz-Wiedemann, N.; Von Krempelhuber, A.; Uebler, N.; Vollmar, J.; Virgin, G.; Chaplin, P. Cardiac Safety of Modified Vaccinia Ankara for Vaccination against Smallpox in a Young, Healthy Study Population. PLoS ONE 2015, 10, e0122653. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, R.N.; Hay, C.M.; Stapleton, J.T.; Marbury, T.C.; Wagner, E.; Kreitmeir, E.; Röesch, S.; Von Krempelhuber, A.; Young, P.; Nichols, R.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase II Trial Investigating the Safety and Immunogenicity of Modified Vaccinia Ankara Smallpox Vaccine (MVA-BN®) in 56-80-Year-Old Subjects. PLoS ONE 2016, 11, e0157335. [Google Scholar] [CrossRef]
- Sutter, M.; Meisinger-Henschel, C.; Tzatzaris, M.; Hülsemann, V.; Lukassen, S.; Wulff, N.H.; Hausmann, J.; Howley, P.; Chaplin, P. Modified vaccinia Ankara strains with identical coding sequences actually represent complex mixtures of virus-es that determine the biological properties of each strain. Vaccine 2009, 27, 7442–7450. [Google Scholar] [CrossRef] [PubMed]
- Okeke, M.I.; Okoli, A.S.; Diaz, D.; Offor, C.; Oludotun, T.G.; Tryland, M.; Bøhn, T.; Moens, U. Hazard Characterization of Modified Vaccinia Virus Ankara Vector: What Are the Knowledge Gaps? Viruses 2017, 9, 318. [Google Scholar] [CrossRef]
- Hansen, H.; Okeke, M.I.; Nilssen, O.; Traavik, T. Recombinant viruses obtained from co-infection in vitro with a live vac-cinia-vectored influenza vaccine and a naturally occurring cowpox virus display different plaque phenotypes and loss of the transgene. Vaccine 2004, 23, 499–506. [Google Scholar] [CrossRef]
- Verheust, C.; Goossens, M.; Pauwels, K.; Breyer, D. Biosafety aspects of modified vaccinia virus Ankara (MVA)-based vec-tors used for gene therapy or vaccination. Vaccine 2012, 30, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Earl, P.L.; Americo, J.L.; Wyatt, L.S.; Espenshade, O.; Bassler, J.; Gong, K.; Lin, S.; Peters, E.; Rhodes Jr, L.; Spano, Y.E.; et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl. Acad. Sci. USA 2008, 105, 10889–10894. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; González, J.M.; Climent, N.; Reyburn, H.; López-Fernández, L.A.; Nájera, J.L.; Gómez, C.E.; García, F.; Gatell, J.M.; Gallart, T.; et al. Selective induction of host genes by MVA-B, a candidate vaccine against HIV/AIDS. J. Virol. 2010, 84, 8141–8152. [Google Scholar] [CrossRef]
- Joachim, A.; Nilsson, C.; Aboud, S.; Bakari, M.; Lyamuya, E.F.; Robb, M.L.; Marovich, M.A.; Earl, P.; Moss, B.; Ochsenbauer, C.; et al. Potent functional antibody responses elic-ited by HIV-I DNA priming and boosting with heterologous HIV-1 recombinant MVA in healthy Tanzanian adults. PLoS ONE 2015, 10, e0118486. [Google Scholar]
- Nilsson, C.; Godoy-Ramirez, K.; Hejdeman, B.; Bråve, A.; Gudmundsdotter, L.; Hallengärd, D.; Currier, J.R.; Wieczorek, L.; Hasselrot, K.; Earl, P.L.; et al. Broad and po-tent cellular and humoral immune responses after a second late HIV-modified vaccinia virus ankara vaccination in HIV-DNA-primed and HIV-modified vaccinia virus Ankara-boosted Swedish vaccines. AIDS Res. Hum. Retrovir. 2014, 30, 299–311. [Google Scholar] [CrossRef]
- Tameris, M.D.; Hatherill, M.; Landry, B.S.; Scriba, T.J.; Snowden, M.A.; Lockhart, S.; Shea, J.E.; McClain, J.B.; Hussey, G.D.; Hanekom, W.A.; et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previ-ously vaccinated with BCG: A randomised, placebo-controlled phase 2b trial. Lancet 2013, 381, 1021–1028. [Google Scholar] [CrossRef]
- Hodgson, S.H.; Ewer, K.; Bliss, C.M.; Edwards, N.; Rampling, T.; Anagnostou, N.A.; De Barra, E.; Havelock, T.; Bowyer, G.; Poulton, I.D.; et al. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J. Infect. Dis. 2014, 211, 1076–1086. [Google Scholar] [CrossRef]
- Biswas, S.; Choudhary, P.; Elias, S.; Miura, K.; Milne, K.H.; De Cassan, S.C.; Collins, K.; Halstead, F.; Bliss, C.M.; Ewer, K.; et al. Assessment of humoral immune responses to blood-stage malaria antigens following ChAd63-MVA immunization, controlled human malaria infection and natural exposure. PLoS ONE 2014, 9, e107903. [Google Scholar] [CrossRef]
- Sebastian, S.; Gilbert, S.C. Recombinant modified vaccinia virus Ankara-based malaria vaccines. Expert Rev. Vaccines 2015, 15, 91–103. [Google Scholar] [CrossRef]
- Milligan, I.D.; Gibani, M.M.; Sewell, R.; Clutterbuck, E.A.; Campbell, D.; Plested, E.; Nuthall, E.; Voysey, M.; Silva-Reyes, L.; McElrath, M.J.; et al. Safety and Immunogenicity of Novel Adenovirus Type 26- and Modified Vaccinia Ankara-Vectored Ebola Vaccines: A Randomized Clinical Trial. JAMA 2016, 315, 1610–1623. [Google Scholar] [CrossRef]
- Tapia, M.D.; O Sow, S.; E Lyke, K.; Haidara, F.C.; Diallo, F.; Doumbia, M.; Traore, A.; Coulibaly, F.; Kodio, M.; Onwuchekwa, U.; et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: A phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2015, 16, 31–42. [Google Scholar] [CrossRef]
- Callendret, B.; Vellinga, J.; Wunderlich, K.; Rodriguez, A.; Steigerwald, R.; Dirmeier, U.; Cheminay, C.; Volkmann, A.; Bra-sel, T.; Carrion, R.; et al. A prophylactic multivalent vaccine against different filovirus species is immunogenic and provides protection from lethal infections with Ebolavirus and Marburgvirus species in non-human primates. PLoS ONE 2018, 13, e0192312. [Google Scholar]
- Wagstaffe, H.R.; Clutterbuck, E.A.; Bockstal, V.; Stoop, J.N.; Luhn, K.; Douoguih, M.J.; Shukarev, G.; Snape, M.D.; Pollard, A.J.; Riley, E.M.; et al. Ebola virus glycoprotein stimulates IL-18–dependent natural killer cell responses. J. Clin. Investig. 2020, 130, 3936–3946. [Google Scholar] [CrossRef]
- Fuentes, S.; Ravichandran, S.; Coyle, E.M.; Klenow, L.; Khurana, S. Human Antibody Repertoire following Ebola Virus In-fection and Vaccination. iScience 2020, 23, 100920. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef]
- Jordan, E.; Lawrence, S.J.; Meyer, Y.P.H.; Schmidt, D.; Schultz, S.; Mueller, J.; Stroukova, D.; Koenen, B.; Gruenert, R.; Sil-bernagl, G.; et al. Broad Antibody and Cellular Immune Re-sponse From a Phase 2 Clinical Trial with a Novel Multivalent Poxvirus-Based Respiratory Syncytial Virus Vaccine. J. Infect. Dis. 2021, 223, 1062–1072. [Google Scholar] [CrossRef]
- Koch, T.; Dahlke, C.; Fathi, A.; Kupke, A.; Krähling, V.; A Okba, N.M.; Halwe, S.; Rohde, C.; Eickmann, M.; Volz, A.; et al. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: An open-label, phase 1 trial. Lancet Infect. Dis. 2020, 20, 827–838. [Google Scholar] [CrossRef]
- Aldoss, I.; La Rosa, C.; Baden, L.R.; Longmate, J.; Ariza-Heredia, E.J.; Rida, W.N.; Lingaraju, C.R.; Zhou, Q.; Martinez, J.; Kaltcheva, T.; et al. Poxvirus Vectored Cytomegalovirus Vaccine to Prevent Cytomegalovirus Viremia in Transplant Recipients. Ann. Intern. Med. 2020, 172, 306. [Google Scholar] [CrossRef]
- Kreijtz, J.H.; Goeijenbier, M.; Moesker, F.M.; van den Dries, L.; Goeijenbier, S.; De Gruyter, H.; Lehmann, M.H.; de Mutsert, G.; van de Vijver, D.; Volz, A.; et al. Safety and immu-nogenicity of a modified-vaccinia-virus-Ankara-based influenza A H5N1 vaccine: A randomised, double-blind phase 1/2a clinical trial. Lancet Infect. Dis. 2014, 14, 1196–1207. [Google Scholar] [CrossRef]
- Lillie, P.J.; Berthoud, T.K.; Powell, T.J.; Lambe, T.; Mullarkey, C.; Spencer, A.J.; Hamill, M.; Peng, Y.; Blais, M.-E.; Duncan, C.; et al. Prelimi-nary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin. Infect. Dis. 2012, 55, 19–25. [Google Scholar] [CrossRef]
- Puksuriwong, S.; Ahmed, M.S.; Sharma, R.; Krishnan, M.; Leong, S.; Lambe, T.; McNamara, P.S.; Gilbert, S.C.; Zhang, Q. Modified Vaccinia Ankara–Vectored Vaccine Expressing Nucleoprotein and Matrix Protein 1 (M1) Activates Mucosal M1-Specific T-Cell Immunity and Tissue-Resident Memory T Cells in Human Nasopharynx-Associated Lymphoid Tissue. J. Infect. Dis. 2019, 222, 807–819. [Google Scholar] [CrossRef]
- Volz, A.; Sutter, G. Protective efficacy of Modified Vaccinia virus Ankara in preclinical studies. Vaccine 2013, 31, 4235–4240. [Google Scholar] [CrossRef]
- Alberca, B.; Bachanek-Bankowska, K.; Cabana, M.; Calvo-Pinilla, E.; Viaplana, E.; Frost, L.; Gubbins, S.; Urniza, A.; Mertens, P.; Castillo-Olivares, J. Vaccination of horses with a recombinant modified vaccinia Ankara virus (MVA) expressing African horse sickness (AHS) virus major capsid protein VP2 provides complete clinical protection against challenge. Vaccine 2014, 32, 3670–3674. [Google Scholar] [CrossRef]
- Haagmans, B.L.; van den Brand, J.; Raj, V.S.; Volz, A.; Wohlsein, P.; Smits, S.L.; Schipper, D.; Bestebroer, T.M.; Okba, N.; Fux, R.; et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science 2016, 351, 77–81. [Google Scholar] [CrossRef]
- Lopera-Madrid, J.; Osorio, J.E.; He, Y.; Xiang, Z.; Adams, L.G.; Laughlin, L.C.; Mwangi, W.; Subramanya, S.; Neilan, J.; Brake, D.; et al. Safety and immunogenicity of mammalian cell de-rived and Modified Vaccinia Ankara vectored African swine fever subunit antigens in swine. Veter Immunol. Immunopathol. 2017, 185, 20–33. [Google Scholar] [CrossRef]
- Zajac, M.; Zanetti, F.A.; Esusy, M.S.; Federico, C.R.; Zabal, O.; Valera, A.R.; Calamante, G. Induction of Both Local Immune Response in Mice and Protection in a Rabbit Model by Intranasal Immunization with Modified Vaccinia Ankara Virus Ex-pressing a Secreted Form of Bovine Herpesvirus 1 Glycoprotein D. Viral Immunol. 2017, 30, 70–76. [Google Scholar] [CrossRef]
- Lorenzo, G.; López-Gil, E.; Ortego, J.; Brun, A. Efficacy of different DNA and MVA prime-boost vaccination regimens against a Rift Valley fever virus (RVFV) challenge in sheep 12 weeks following vaccination. Veter Res. 2018, 49, 1–12. [Google Scholar] [CrossRef]
- Volz, A.; Fux, R.; Langenmayer, M.C.; Sutter, G. Modified vaccinia virus ankara (MVA)—development as recombinant vac-cine and prospects for use in veterinary medicine. Berl. Munch Tierarztl. Wochenschr. 2015, 128, 464–472. [Google Scholar]
- Marín-López, A.; Barreiro-Piñeiro, N.; Utrilla-Trigo, S.; Barrialesa, D.; Benavente, J.; Nogalesa, A.; Martínez-Costas, J.; Orte-go, J.; Calvo-Pinilla, E. Cross-protective immune responses against African horse sickness virus after vaccination with pro-tein NS1 delivered by avian reovirus muNS microspheres and modified vaccinia virus Ankara. Vaccine 2020, 38, 882–889. [Google Scholar] [CrossRef]
- Calvo-Pinilla, E.; Marín-López, A.; Moreno, S.; Lorenzo, G.; Utrilla-Trigo, S.; Jiménez-Cabello, L.; Benavides, J.; Nogales, A.; Blasco, R.; Brun, A.; et al. A protective bivalent vaccine against Rift Valley fever and bluetongue npj. Vaccines 2020, 5, 1–12. [Google Scholar] [CrossRef]
- Prkno, A.; Hoffmann, D.; Kaiser, M.; Goerigk, D.; Pfeffer, M.; Winter, K.; Vahlenkamp, T.W.; Beer, M.; Starke, A. Field Trial Vaccination against Cowpox in Two Alpaca Herds. Viruses 2020, 12, 234. [Google Scholar] [CrossRef]
- Trigo, S.U.; Jiménez-Cabello, L.; Alonso-Ravelo, R.; Calvo-Pinilla, E.; Marín-López, A.; Moreno, S.; Lorenzo, G.; Benavides, J.; Gilbert, S.; Nogales, A.; et al. Heterologous Combination of ChAdOx1 and MVA Vectors Expressing Protein NS1 as Vaccination Strategy to Induce Durable and Cross-Protective CD8+ T Cell Immunity to Bluetongue Virus. Vaccines 2020, 8, 346. [Google Scholar] [CrossRef]
- García, F.; de Quirós, J.C.; Gómez, C.E.; Perdiguero, B.; Nájera, J.L.; Jiménez, V.; García-Arriaza, J.; Guardo, A.C.; Pérez, I.; Díaz-Brito, V.; et al. Safety and immunogen-icity of a modified pox vector-based HIV/AIDS vaccine candidate expressing Env, Gag, Pol and Nef proteins of HIV-1 sub-type B (MVA-B) in healthy HIV-1-uninfected volunteers: A phase I clinical trial (RISVAC02). Vaccine 2011, 29, 8309–8316. [Google Scholar] [CrossRef]
- Gómez, C.E.; Nájera, J.L.; Perdiguero, B.; García-Arriaza, J.; Sorzano, C.O.S.; Jiménez, V.; González-Sanz, R.; Jiménez, J.L.; Muñoz-Fernández, M.A.; de Quirós, J.C.L.B.; et al. The HIV/AIDS Vaccine Candidate MVA-B Administered as a Single Immunogen in Humans Triggers Robust, Polyfunctional, and Selective Effector Memory T Cell Responses to HIV-1 Antigens. J. Virol. 2011, 85, 11468–11478. [Google Scholar] [CrossRef]
- Mothe, B.; Climent, N.; Plana, M.; Rosàs, M.; Jiménez, J.L.; Muñoz-Fernández, M.A.; Puertas, M.C.; Carrillo, J.; Gonzalez, N.; León, A.; et al. RISVAC-03 Study Group. Safety and immunogenicity of a modified vaccinia Ankara-based HIV-1 vaccine (MVA-B) in HIV-1-infected patients alone or in combination with a drug to reactivate latent HIV-1. J. Antimicrob. Chemother 2015, 70, 1833–1842. [Google Scholar]
- Pierantoni, A.; Esposito, M.L.; Ammendola, V.; Napolitano, F.; Grazioli, F.; Abbate, A.; del Sorbo, M.; Siani, L.; D’Alise, A.M.; Taglioni, A.; et al. Mucosal delivery of a vectored RSV vaccine is safe and elicits protective immunity in rodents and nonhuman primates. Mol. Ther. Methods Clin. Dev. 2015, 2, 15018. [Google Scholar] [CrossRef]
- Russell, M.S.; Thulasi Raman, S.N.; Gravel, C. Single Immunization of a Vaccine Vectored by a Novel Recombinant Vac-cinia Virus Affords Effective Protection Against Respiratory Syncytial Virus Infection in Cotton Rats. Front. Immunol. 2021, 12, 747866. [Google Scholar] [CrossRef]
- Samy, N.; Reichhardt, D.; Schmidt, D.; Chen, L.M.; Silbernagl, G.; Vidojkovic, S.; Meyer, T.P.; Jordan, E.; Adams, T.; Wei-denthaler, H.; et al. Safety and immunogenicity of novel modified vaccinia Anka-ra-vectored RSV vaccine: A randomized phase I clinical trial. Vaccine 2020, 38, 2608–2619. [Google Scholar] [CrossRef]
- Prabakaran, M.; Leyrer, S.; He, F.; Auer, S.; Kumar, S.R.; Kindsmueller, K.; Mytle, N.; Schneider, J.; Lockhart, S.; Kwang, J. Progress toward a Universal H5N1 Vaccine: A Recombinant Modified Vaccinia Virus Ankara-Expressing Trivalent Hemag-glutinin Vaccine. PLoS ONE 2014, 9, e107316. [Google Scholar] [CrossRef]
- Berthoud, T.K.; Hamill, M.; Lillie, P.J.; Hwenda, L.; Collins, K.A.; Ewer, K.J.; Milicic, A.; Poyntz, H.C.; Lambe, T.; Fletcher, H.A.; et al. Potent CD8+ T-Cell Immunogenicity in Humans of a Novel Heterosubtypic Influenza A Vac-cine, MVA−NP+M1. Clin. Infect. Dis. 2011, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Antrobus, R.D.; Lillie, P.; Berthoud, T.K.; Spencer, A.; McLaren, J.; Ladell, K.; Lambe, T.; Milicic, A.; Price, D.; Hill, A.V.S.; et al. A T Cell-Inducing Influenza Vaccine for the Elderly: Safety and Immunogenicity of MVA-NP+M1 in Adults Aged over 50 Years. PLoS ONE 2012, 7, e48322. [Google Scholar] [CrossRef] [PubMed]
- Mintaev, R.R.; Glazkova, D.V.; Orlova, O.V.; Bogoslovskaya, E.V.; Shipulin, G.A. Development of a Universal Epitope-Based Influenza Vaccine and Evaluation of Its Effectiveness in Mice. Vaccines 2022, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Volz, A.; Kupke, A.; Song, F. Protective efficacy of recombinant modified vaccinia virus Ankara delivering Middle East res-piratory syndrome coronavirus spike glycoprotein. J. Virol. 2015, 89, 8651–8656. [Google Scholar] [CrossRef]
- Chiuppesi, F.; d’Alincourt Salazar, M.; Contreras, H.; Nguyen, V.H.; Martinez, J.; Park, Y.; Nguyen, J.; Kha, M.; Iniguez, A.; Zhou, Q.; et al. Development of a multi-antigenic SARS-CoV-2 vaccine candidate using a synthetic poxvirus platform. Nat. Commun. 2020, 11, e6121. [Google Scholar] [CrossRef]
- Chiuppesi, F.; Nguyen, C.H.; Park, Y.; Contreras, H.; Karpinski, V.; Faircloth, K.; Nguyen, J.; Kha, M.; Johnson, D.; Mar-tinez, J.; et al. Synthetic multiantigen MVA vaccine COH04S1 protects against SARS-CoV-2 in Syrian hamsters and non-human primates npj. Vaccines 2022, 7, 7. [Google Scholar]
- Schäfer, B.; Holzer, G.W.; Joachimsthaler, A.; Coulibaly, S.; Schwendinger, M.; Crowe, B.A.; Kreil, T.R.; Barrett, P.N.; Falkner, F.G. Pre-Clinical Efficacy and Safety of Experimental Vaccines Based on Non-Replicating Vaccinia Vectors against Yellow Fever. PLoS ONE 2011, 6, e24505. [Google Scholar] [CrossRef]
- Weger-Lucarelli, J.; Chu, H.; Aliota, M.; Partidos, C.D.; Osorio, J.E. A Novel MVA Vectored Chikungunya Virus Vaccine Elicits Protective Immunity in Mice. PLoS Neglected Trop. Dis. 2014, 8, e2970. [Google Scholar] [CrossRef]
- Bošnjak, B.; Odak, I.; Barros-Martins, J.; Sandrock, I.; Hammerschmidt, S.I.; Permanyer, M.; Patzer, G.E.; Greorgiev, H.; Jau-regui, R.G.; Tscherne, A.; et al. Intranasal Delivery of MVA Vector Vaccine Induces Effective Pulmonary Immunity Against SARS-CoV-2 in Rodents. Front. Immunol. 2021, 12, e7722240. [Google Scholar] [CrossRef]
- Jeffrey, L.; Americo, J.L.; Cotter, C.A.; Earl, P.L.; Liu, R.; Moss, B. Intranasal inoculation of an MVA-based vaccine induces IgA and protects the respiratory tract of hACE2 mice from SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2022, 119, e2202069119. [Google Scholar]
- Bender, B.S.; Rowe, C.A.; Taylor, S.F.; Wyatt, L.S.; Moss, B.; Small, P.A. Oral Immunization with a replication-deficient re-combinant vaccinia virus protects mice against influenza. J. Virol. 1996, 70, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Xia, H.; Adam, A.; Wang, B.; Hajnik, R.H.; Liang, Y.; Rafael, G.H.; Zou, J.; Wang, X.; Sun, J.; et al. Mucosal vaccination induces protection against SARS-CoV-2 in the absence of detectable neutralizing antibodies. NPJ Vaccines 2021, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Thomas, Z.-R.M.; Satti, I.; Marshall, J.L.; Harris, S.A.; Ramon, R.L.; Hamidi, A.; Minhinnick, A.; Riste, M.; Stockdale, L.; Lawrie, A.M.; et al. Alternate aerosol and systemic immunisation with a recombinant viral vector for tuberculosis, MVA85A: A phase I randomised controlled trial. PLoS Med. 2019, 16, e1002790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satti, I.; Meyer, J.; Harris, S.A.; Thomas, Z.-R.M.; Griffiths, K.; Antrobus, R.D.; Rowland, R.; Ramon, R.L.; Smith, M.; Sheehan, S.; et al. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: A phase 1, double-blind, randomised controlled trial. Lancet Infect. Dis. 2014, 14, 939–946. [Google Scholar] [CrossRef]
- Moss, B.; Carroll, M.W.; Wyatt, L.S.; Bennink, J.R.; Hirsch, V.M.; Goldstein, S.; Elkins, W.R.; Fuerst, T.R.; Lifson, J.D.; Pi-atak, M.; et al. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv. Exp. Med. Biol. 1996, 397, 7–13. [Google Scholar]
- Harrop, R.; Connolly, N.; Redchenko, I.; Valle, J.; Saunders, M.; Ryan, M.G.; Myers, K.A.; Drury, N.; Kingsman, S.M.; Hawkins, R.E.; et al. Vaccination of Colorectal Cancer Patients with Modified Vaccinia Ankara Delivering the Tumor Antigen 5T4 (TroVax) Induces Immune Responses which Correlate with Disease Control: A Phase I/II Trial. Clin. Cancer Res. 2006, 12, 3416–3424. [Google Scholar] [CrossRef]
- Cottingham, M.G.; Carroll, M.W. Recombinant MVA vaccines: Dispelling the myths. Vaccine 2013, 31, 4247–4251. [Google Scholar] [CrossRef]
- Harrop, R.; Shingler, W.; Kelleher, M.; de Belin, J.; Treasure, P. Cross-trial analysis of immunologic and clinical data result-ing from phase I and II trials of MVA- 5T4 (TroVax) in colorectal, renal, and prostate cancer patients. J. Immunother 2010, 33, 999–1005. [Google Scholar] [CrossRef]
- Goepfert, P.A.; Elizaga, M.L.; Sato, A.; Qin, L.; Cardinali, M.; Hay, C.M.; Hural, J.; DeRosa, S.C.; Defawe, O.D.; Tomaras, G.D.; et al. Phase 1 Safety and Immunogenicity Testing of DNA and Recombinant Modified Vaccinia Ankara Vaccines Expressing HIV-1 Virus-like Particles. J. Infect. Dis. 2011, 203, 610–619. [Google Scholar] [CrossRef]
- Altenburg, A.F.; van Trierum, S.E.; de Bruin, E.; de Meulder, D.; van de Sandt, K.E.; van der Klis, F.; Fouchier, R.; Koopmans, M.; Rimmelzwaan, G.F.; de Vries, R.D. Effects of pre-existing orthopoxvirus-specific immunity on the perfor-mance of Modified Vaccinia virus Ankara-based influenza vaccines. Sci. Rep. 2018, 8, 6474. [Google Scholar] [CrossRef]
- Ba, L.; Yi, C.E.; Zhang, L.; Ho, D.D.; Chen, Z. Heterologous MVA-S prime Ad5-S boost regimen induces high and persistent levels of neutralizing antibody response against SARS coronavirus. Appl. Microbiol. Biotechnol. 2007, 76, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Chiuppesi, F.; Wussow, F.; Scharf, L.; Contreras, H.; Gao, H.; Meng, Z.; Nguyen, J.; Barry, P.A.; Bjorkman, P.J.; Diamond, D.J. Comparison of homologous and heterologous prime-boost vaccine approaches using Modified Vaccinia Ankara and sol-uble protein to induce neutralizing antibodies by the human cytomegalovirus pentamer complex in mice. PLoS ONE 2017, 12, e0183377. [Google Scholar] [CrossRef] [PubMed]
- Ewer, K.J.; O’Hara, G.A.; Duncan, C.; Collins, K.A.; Sheehy, S.H.; Reyes-Sandoval, A.; Goodman, A.L.; Edwards, N.J.; Elias, S.C.; Halstead, F.D.; et al. Pro-tective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunization. Nat. Commun. 2013, 4, 2836. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Gilbert, S.C.; Blanchard, T.J.; Hanke, T.; Robson, K.J.; Hannan, C.M.; Becker, M.; Sinden, R.E. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 1998, 4, 397–402. [Google Scholar] [CrossRef]
- Coughlan, L.; Sridhar, S.; Payne, R.; Edmans, M.; Milicic, A.; Venkatraman, N.; Lugonja, B.; Clifton, L.; Qi, C.; Folegatti, P.M.; et al. Heterologous Two-Dose Vaccination with Simian Adenovirus and Poxvirus Vectors Elicits Long-Lasting Cellular Immunity to Influenza Virus A in Healthy Adults. Lancet 2018, 29, 146–154. [Google Scholar] [CrossRef]
- Colby, D.J.; Sarnecki, M.; Barouch, D.H.; Tipsuk, S.; Stieh, D.J.; Kroon, E.; Schuetz, A.; Intasan, J.; Sacdalan, C.; Pinyakorn, S.; et al. Safety and immunogenicity of Ad26 and MVA vaccines in acutely treated HIV and effect on viral rebound after antiretroviral therapy interruption. Nat. Med. 2020, 26, 498–501. [Google Scholar] [CrossRef]
- Venkatraman, N.; Ndiaye, B.P.; Bowyer, G.; Wade, D.; Sridhar, S.; Wright, D.; Powlson, J.; Ndiaye, I.; Dièye, S.; Thompson, C.; et al. Safety and Immunogenicity of a Heterologous Prime-Boost Ebola Virus Vaccine Regimen in Healthy Adults in the United Kingdom and Senegal. J. Infect. Dis. 2018, 219, 1187–1197. [Google Scholar] [CrossRef]
- Mutua, G.; Anzala, O.; Luhn, K.; Robinson, C.; Bockstal, V.; Anumendem, D.; Douoguih, M. Safety and immunogenicity of a 2-dose heterologous vaccine regimen with Ad26.ZEBOV and MVA-BN-Filo Ebola vaccines: 12-month data from a Phase 1 randomized clinical trial in Nairobi, Kenya. J. Infect. Dis. 2019, 220, 57–67. [Google Scholar] [CrossRef]
- Anywaine, Z.; Barry, H.; Anzala, O.; Mutua, G.; Sirima, S.B.; Eholie, S.; Kibuuka, H.; Bétard, C.; Richert, L.; Lacabaratz, C.; et al. Safety and immunogenicity of 2-dose heterologous Ad26.ZEBOV, MVA-BN-Filo Ebola vaccination in children and adolescents in Africa: A randomised, placebo-controlled, multicentre Phase II clinical trial. PLoS Med. 2022, 19, e1003865. [Google Scholar] [CrossRef]
- Payne, R.O.; Silk, S.E.; Elias, S.; Milne, K.H.; Rawlinson, T.A.; Llewellyn, D.; Shakri, A.R.; Jin, J.; Labbé, G.M.; Edwards, N.J.; et al. Human vaccination against Plasmodium vivax Duffy-binding protein induces strain-transcending antibodies. JCI Insight 2017, 2, 93683. [Google Scholar] [CrossRef] [Green Version]
| VACV Strain | Country or Region of Application |
|---|---|
| New York City Board of Health (NYCBOH), Dryvax | USA |
| Lister | UK, Europe, Asia, Africa, USA |
| Temple of Heaven (Tian Tan) | China |
| Tashkent, Gam, MRIVP, Per, B-51 | USSR |
| Lister/L-IVP | USSR/Russian Federation |
| Bern | Germany, Austria |
| Paris | France, Syria, Turkey |
| Copenhagen | Denmark |
| Dairen, Ikeda | Japan |
| Sweden | Sweden |
| Ankara | Turkey |
| Chambon | France and Africa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlova, O.V.; Glazkova, D.V.; Bogoslovskaya, E.V.; Shipulin, G.A.; Yudin, S.M. Development of Modified Vaccinia Virus Ankara-Based Vaccines: Advantages and Applications. Vaccines 2022, 10, 1516. https://doi.org/10.3390/vaccines10091516
Orlova OV, Glazkova DV, Bogoslovskaya EV, Shipulin GA, Yudin SM. Development of Modified Vaccinia Virus Ankara-Based Vaccines: Advantages and Applications. Vaccines. 2022; 10(9):1516. https://doi.org/10.3390/vaccines10091516
Chicago/Turabian StyleOrlova, Olga Vladimirovna, Dina Viktorovna Glazkova, Elena Vladimirovna Bogoslovskaya, German Alexandrovich Shipulin, and Sergey Mikhailovich Yudin. 2022. "Development of Modified Vaccinia Virus Ankara-Based Vaccines: Advantages and Applications" Vaccines 10, no. 9: 1516. https://doi.org/10.3390/vaccines10091516





